Optimal cartilage repair in the knee one step closer through second phase of clinical study Hy2Care’s CartRevive™
Geleen, March 30, 2023
Optimal cartilage repair in the knee one step closer through second phase of clinical study
Hy2Care’s CartRevive™ hydrogel implant designed to improve cartilage repair surgeries
Hy2Care announced today that it will initiate the next phase of its ACTIVE clinical study to improve cartilage repair of the knee joint. After completing the first ‘safety’ group of 10 patients early 2023, the Medical Ethical Review Committee of the UMC Utrecht has granted approval to continue the clinical study with the next group of 36 patients. The trial will be extended to include also the Maastricht UMC+ and the Elisabeth TweeSteden Ziekenhuis in Tilburg, the Netherlands.
The CartRevive™ hydrogel implant has been developed to enable optimal cartilage repair in the knee. This addresses a large unmet clinical need based on the high prevalence of cartilage repair surgeries worldwide.
Promising solution for many patients
Articular cartilage is easily damaged but hard to regenerate. Damage is caused by sports injuries, accidents, slips and falls. An estimated 2.5 million TFD Traumatic Focal Defect cases are diagnosed each year worldwide. Unfortunately, some of the (current) suboptimal surgeries will only relieve patients of their discomforts for a limited amount of time, the return of symptoms causing many years of pain and disabilities. Orthopedic surgeon and ACTIVE study principal investigator Roel Custers from the UMC Utrecht expresses high hopes for the new approach: “The development of Hy2Care’s product can be a viable solution for a significant part of the patients for who currently only suboptimal treatment options are available.” Hy2Care aims to reach many patients with the new technology. “Our CartRevive™ hydrogel implant has been designed with the goal to support the body to heal itself. We expect to replace several surgeries which are currently used, with our new hydrogel treatment and ultimately make a positive change in the lives of millions of people”, says Leo Smit, CEO of Hy2Care.
How it works
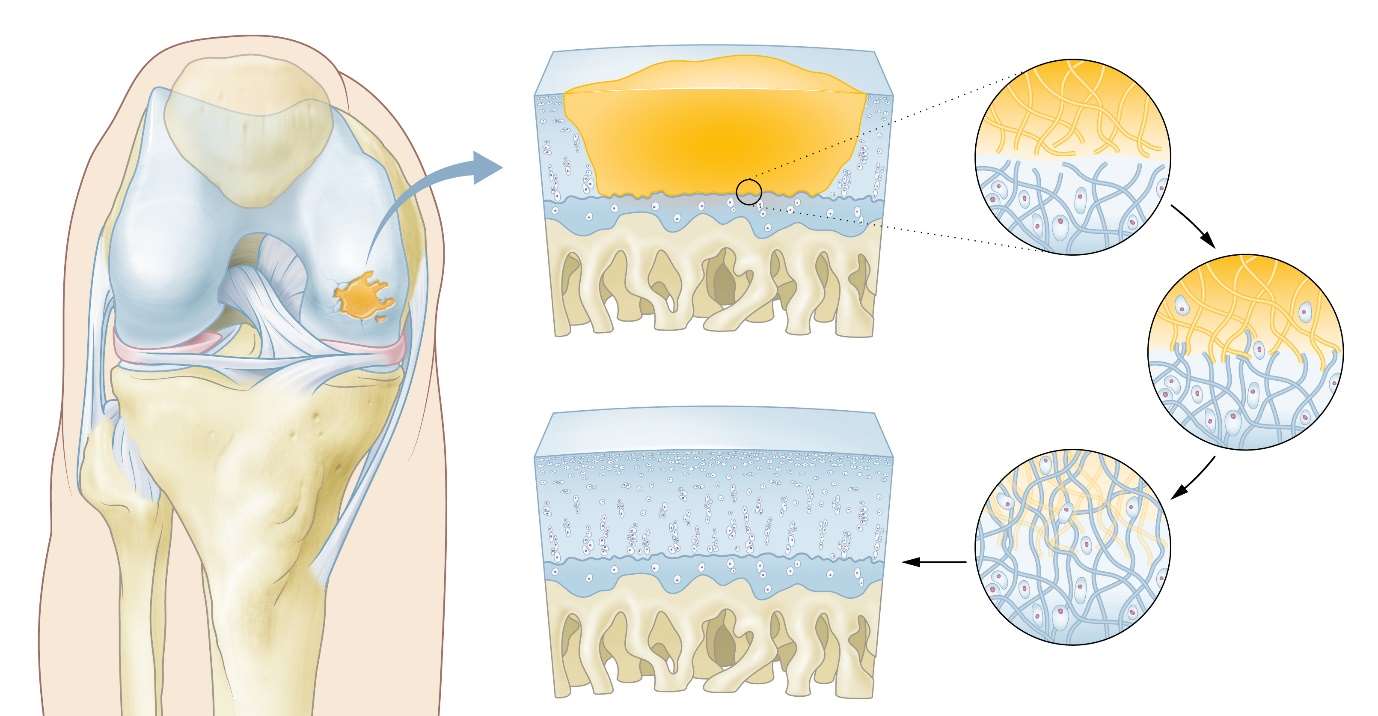
The CartRevive™ hydrogel implant from Hy2Care has been developed for functional recovery of cartilage defects in joints. It is based on joint-tissue mimicking natural polymers; it aims to protect the injured site and allow patient’s own cells to enable cartilage recovery. The easy-flowing gel completely fills the defect which prevents fibrous tissue to enter. The gel covalently binds itself to the surrounding tissue.
The natural polymer-gel gradually degrades, supporting the patient’s own chondrocytes to enter and start the creation of improved Hyaline-like cartilage.
In short: in-situ autologous regeneration: ‘the self-healing knee’.
About Hy2Care
Hy2Care® is a ‘spin-off company’ of the Tech Med Centre of the University of Twente, the Netherlands and was founded in 2014. The original founders, prof. dr. Marcel Karperien and dr. Sanne Both, continue to be active in the company. The unique technology of Hy2Care was developed by prof. dr. Marcel Karperien and his team of the Developmental BioEngineering group at the University of Twente. This hydrogel implant technology is protected through several patents.
Hy2Care’s launching product, the CartRevive™ hydrogel implant for cartilage repair in the knee, is currently clinically investigated in the Netherlands for European market approval. A US clinical trial is in preparation.
The company continues to use facilities in Enschede (NL) at the site of the University of Twente, and has its own laboratory and offices at the Brightlands Chemelot Campus in Geleen.
In 2019 Hy2Care received a €3.7M Series-A investment. It’s current Chief Executive Officer Leo Smit joined, and an additional team expansion was initiated, bringing in also Sanna Severins as Chief Operations Officer and co-director of the company. The goal of the Series-A Investment was to finance the scale up of the technology and initiate clinical trials. Also, a separate product and venture for veterinary applications is under development.
In 2022 the European Commission has awarded Hy2Care €6 million in blended financing. This enables Hy2Care to complete its clinical studies and to obtain European market approval with its game-changing hydrogel implant for optimal cartilage repair. The company receives the financing via the European Innovation Council (EIC) Accelerator program.
About the ACTIVE Clinical Investigation
The name of the study, “ACTIVE”, stands for Advanced Cartilage Treatment with Injectable hydrogel Validation of the Effect. To be eligible to participate in this study patients must meet certain criteria, amongst which medical history, an age between 18 – 50 years, and cartilage trauma with a size of 0.5 – 2cm2. In the surgery, the surgeon will apply the CartRevive™ hydrogel implant to the cartilage defect, which will firmly attach to the surrounding cartilage and bone in less than a minute. After implantation, patients will be periodically checked and examined during the course of 1 year. At this moment the phase 2 study starts including 36 patients to evaluate product performance.
Participating surgeons and hospitals:
UMC Utrecht, dr. Roel Custers, dr. Nienke van Egmond
Maastricht UMC+, dr. Pieter Emans, dr. Tim Boymans
Elisabeth TweeSteden Ziekenhuis Tilburg, dr. Jacob Caron, dr. Chris van den Broek
Leo Smit, CEO at Hy2Care: “Our CartRevive™ hydrogel implant has been designed with the goal to support the body to heal itself. We expect to replace several surgeries which are currently used, with our new hydrogel treatment and ultimately make a positive change in the lives of millions of people.”
Dr. Roel Custers, Orthopedic surgeon at UMC Utrecht: “The development of Hy2Care’s product can be a viable solution for a significant part of the patients for who currently only suboptimal treatment options are available.”
