Geleen, 17 April 2025
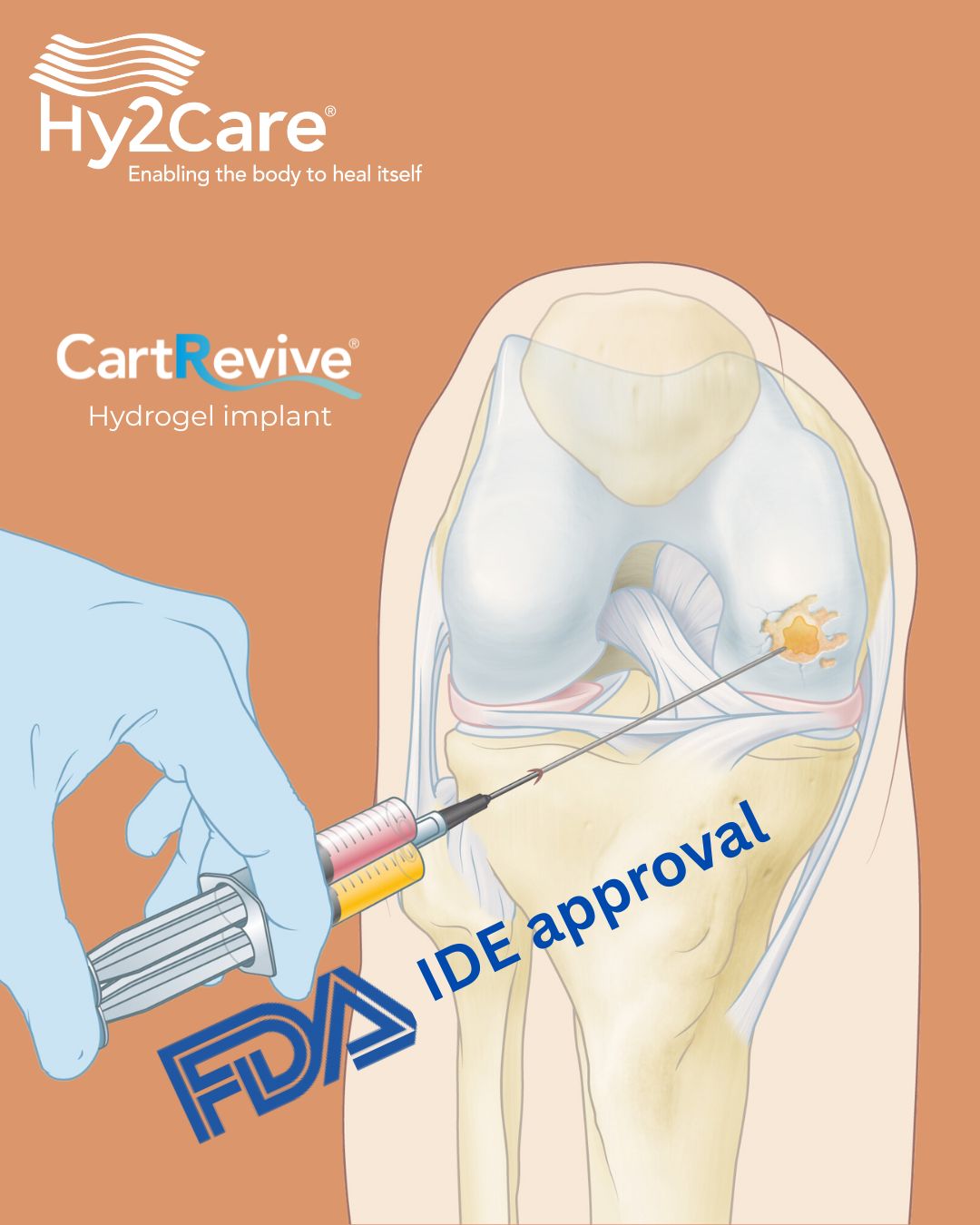
Hy2Care® has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA), clearing the way to initiate its first clinical trial in the United States. This milestone enables the evaluation of Hy2Care’s CartRevive® hydrogel implant, designed to enable optimal repair of traumatic cartilage injuries in the knee, addressing a major unmet clinical need.
The IDE approval builds on the positive results of Hy2Care’s ongoing European clinical trial and highlights the growing recognition of CartRevive® as a unique, minimally invasive solution for orthopedic surgeons and their patients.
Leo Smit, CEO of Hy2Care®, on the milestone:
“Receiving IDE approval is a significant achievement for Hy2Care®. It reflects the strength of our technology, the dedication of our team, and the clinical evidence we have built over the past years. This is not only an important US regulatory step — it is a major step forward in fulfilling our mission of bringing Hy2Care® CartRevive® hydrogel implant to patients worldwide.”
Preparations for the US trial are already underway, with the first patient treatment expected early 2026. The study will be conducted in collaboration with leading orthopedic centres across the United States and Europe.
Co-founder and former patient Dr. Sanne Both added:
“We are grateful to the FDA for the smooth approval process. We have been working closely with the agency — first to obtain Breakthrough Device Designation, and now IDE approval. Considering the agency’s high workload, we are truly pleased to have received this approval at this time.”
Next Steps: U.S. Trial and EU CE Marking
- U.S. clinical trial (following IDE approval) to begin in early 2026
- Conducted in partnership with top-tier orthopedic clinics
- CE marking targeted for 2026, with ongoing efforts toward regulatory approval and commercial launch in Europe
About Hy2Care®
Hy2Care® is a spin-off from the TechMed Centre at the University of Twente (NL), founded in 2014. Founders Prof. Dr. Marcel Karperien and Dr. Sanne Both remain active in the company. In 2022, Hy2Care® received the prestigious EIC (European Innovation Council) Accelerator award to support the market introduction of its innovative hydrogel technology. Its CartRevive® hydrogel implant clinical trial has successfully completed patient enrolment for its EU trial in 2024 and CE marking / European market approval is anticipated by early 2026. CartRevive® was granted FDA Breakthrough Device Designation in 2023 and in April 2025, Hy2Care® received FDA IDE approval to start its first clinical trial in the United States.
Media contact
Wendy Mertens: wendy.mertens@hy2care.com
